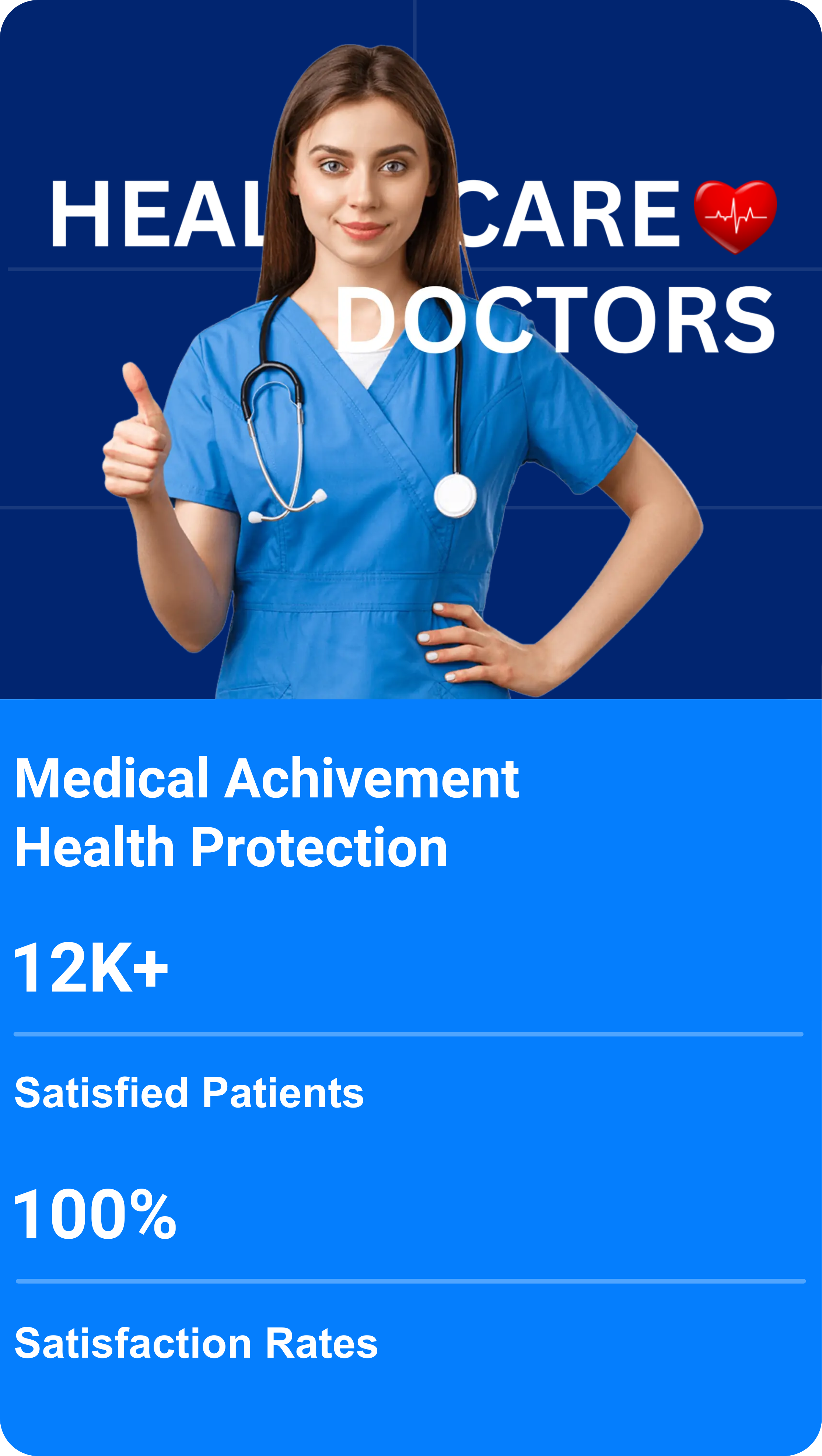
Trial Site Management India


India's Trusted Clinical Trial Site Management Organization
0
+
Patients recruited
0
+
Clinical Projects completed
Our vision
Timely Support
Expert site management ensuring timely,
cost-effective clinical trial solutions.
Our mission

Uncompromising Service
Fostering a healthier society with quality,
commitment, and timely delivery.
What we do ?

Cost Effective
Expert services ensuring efficient trials and
high-quality research outcomes.

In the past five years, we have
recruited more than 6000+ .
We Simplify Clinical Trial Site Management Across India
ONCOLOGY
⚕
CARDIOLOGY
⚕
DERMATOLOGY
⚕
AUTOIMMUNE DISORDERS
⚕
GASTROINTESTINAL DISORDERS
⚕
MENTAL HEALTH DISORDERS
⚕
ENDOCRINE DISORDERS
⚕
MUSCULOSKELETAL DISORDERS
⚕
OBESITY AND WEIGHT MANAGEMENT
⚕
SKIN DISORDERS
⚕
HEMATOLOGIC DISORDERS
⚕
PEDIATRIC CONDITIONS
⚕
PREGNANCY AND REPRODUCTIVE HEALTH
⚕
EYE CONDITIONS
⚕
PAIN MANAGEMENT
⚕
GENETIC DISORDERS
⚕
ONCOLOGY
⚕
CARDIOLOGY
⚕
DERMATOLOGY
⚕
AUTOIMMUNE DISORDERS
⚕
GASTROINTESTINAL DISORDERS
⚕
MENTAL HEALTH DISORDERS
⚕
ENDOCRINE DISORDERS
⚕
MUSCULOSKELETAL DISORDERS
⚕
OBESITY AND WEIGHT MANAGEMENT
⚕
SKIN DISORDERS
⚕
HEMATOLOGIC DISORDERS
⚕
PEDIATRIC CONDITIONS
⚕
PREGNANCY AND REPRODUCTIVE HEALTH
⚕
EYE CONDITIONS
⚕
PAIN MANAGEMENT
⚕
GENETIC DISORDERS
⚕
Phase of clinical trials
Best Clinical Trial Site Management Organization in India and Our team is passionate about providing top-notch site management clinical trial services to contract research organizations (CROs), pharmaceutical companies, biotechnology companies, medical device companies, etc. We have top notch experience in clinical research; we completed good number of clinical trials. Our research sites have all the facilities and a dedicated team to ensure the smooth conduct of clinical trials. We are committed to ethical and compliant practices and prioritize data integrity and confidentiality.
No Data Found
No Data Found
Testimonials



























Trusted by Leading Clinical Trial Organizations in India