About Our Clinical Trial Site Management Organization in India
Clinical Trial Site Management Experts in India
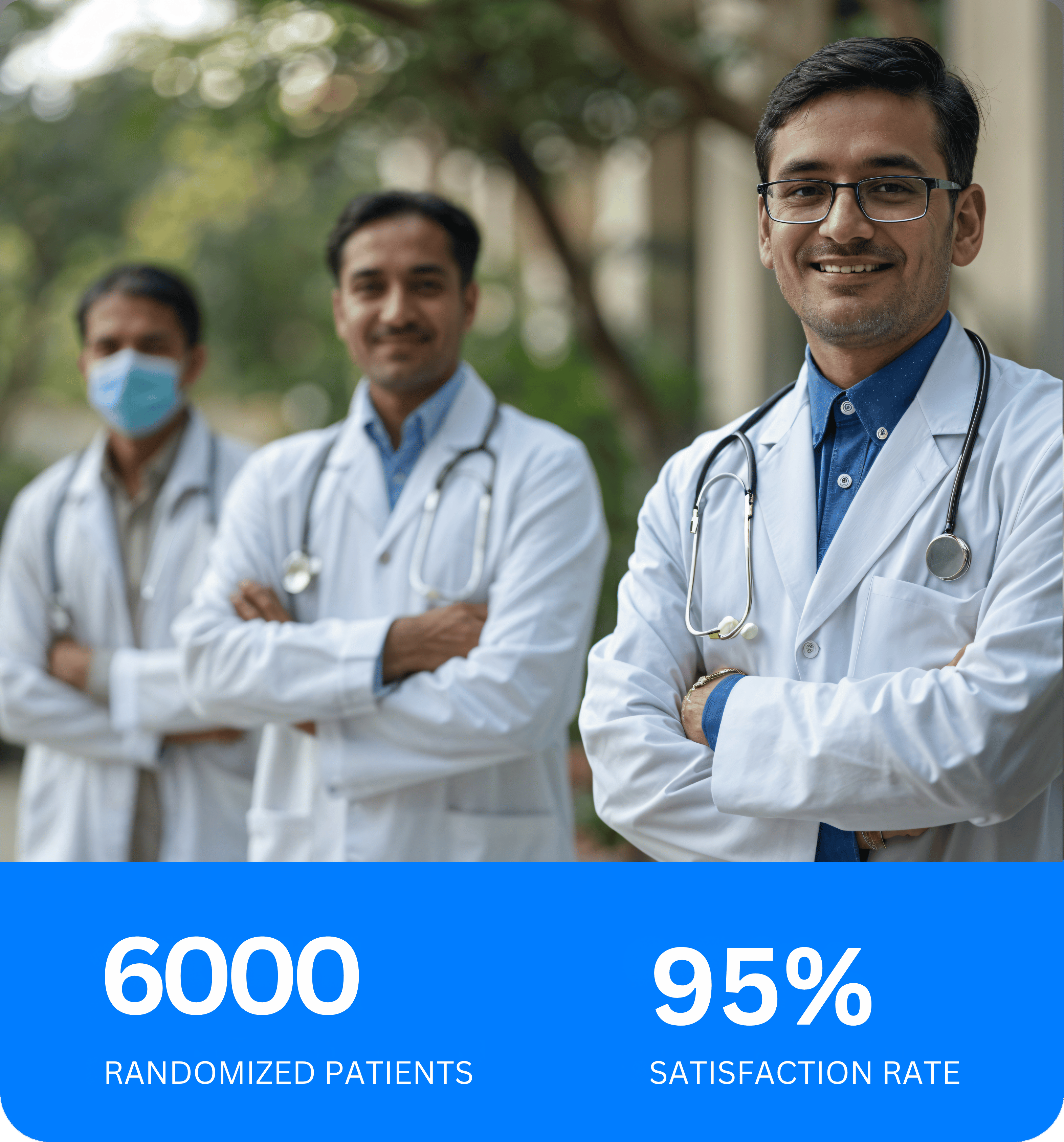
Since 2020, we have provided clinical trial services to clients including sponsors, CROs, and principal investigators.
Site selection and activation
Study start-up support
Patient recruitment and retention
Clinical monitoring
Timely query resolution
Data management
Frequent training to avoid PD/PV
Internal auditing prior to monitoring
About us
Oxygen clinical research and services is a site management organization (SMO) offering comprehensive site management services to contract research organizations (cro) and pharmaceutical & / or biotechnology companies.
Our vision
- Oxygen Clinical Research and Services is a leading SMO offering complete site management to CROs and pharma companies.
- Our primary objective is to provide timely and cost-effective support for clinical trials.
- As clinical research sites, we take pride in delivering honest, high-quality, and timely services in an expedited, efficient manner.
Our mission
- We never compromise on the quality of our work and always deliver projects on time.
- We are a GCP-compliant, subject-centered organization committed to building a healthier society.
- Therefore, we strive to create a healthy, supportive workplace while also constantly aiming for excellence in all we do.
Oxygen Clinical Research and Services is a trusted Site Management Organization (SMO) based in India.
What we do ?
Oxygen Clinical Research is a network of high-performing principal investigators. We understand that the success of clinical trials depends primarily on the skill and dedication of our selected physicians. Our investigators have successfully completed 25+ clinical trials. We conduct Phase I–IV trials, BA/BE studies, device trials, and post-marketing research across diverse therapeutic areas.
Oxygen Clinical Research supports its Principal Investigators by:
Enhancing Visibility of Investigators and Sites
Oxygen Clinical Research manages and executes a full therapeutic portfolio on your behalf. We actively represent you and your clinic during the site selection process, ensuring that sponsors recognize your full potential. By advocating for your capabilities and streamlining communication, we help secure more opportunities for your site. Our specialists ensure your investigative site is equipped with the proper knowledge and tools needed to succeed in a competitive pharmaceutical environment.
Screening Study Opportunities & Site Feasibility
Before introducing new studies, Oxygen Clinical Research performs an initial assessment of your centre’s specific needs, patient pool availability, and infrastructure readiness. This helps determine your site’s feasibility and ensures smooth study execution.
Training & Development
We ensure your research staff is trained in the latest regulatory requirements and the most effective tools for implementing ICH/GCP guidelines. Additionally, Oxygen Clinical Research provides training on study protocols and related materials. Our short-term training programs (1–2 days) cover all clinical trial aspects and clarify each research team member’s roles and responsibilities. Contact us for course availability and pricing.
Qualified Staff Placement
Oxygen Clinical Research provides highly trained professionals, including study coordinators and data entry personnel, to support your clinical trials efficiently and reliably.
Standardization of Research Processes
Our innovative, standardized approach enables your centre to manage multiple studies simultaneously with greater accuracy and consistency.
Provision of Investigator Site SOPs
We enhance your research centre’s quality by standardizing procedures through customized SOPs. This ensures consistent performance at all levels and helps meet sponsor expectations.
Regulatory Document Preparation & Equipment Support
We manage the preparation and submission of regulatory documents to the Independent Ethics Committee, CROs, sponsors, and strategic partners. Moreover, Oxygen Clinical Research provides sites with all protocol-required tools, such as ECG machines, spirometers, centrifuges, BP apparatus, and more.
Contract Review & Negotiation
Investigators often lack time to thoroughly review Clinical Trial Agreements (CTAs). As part of our network, we act as your legal representative to ensure fair and favorable contract terms.
Source Document Worksheet Creation
Our team develops study-related source document worksheets. These tools improve information management and ensure data accuracy and completeness in CRFs.
Recruitment Support
Together with investigators, we recruit large numbers of subjects quickly. Since identifying trial subjects can be time-consuming, we use a defined subject selection process. Potential participants are informed through advertisements, referral programs, and awareness campaigns.
Payment Administration & Feasibility Services
We handle budget negotiations with principal investigators, estimate study costs, and perform marketing analysis before trials begin. Our team tracks patient enrollment and visit procedures to ensure accurate and timely sponsor payments. We also reconcile payment data and prevent delays by closely monitoring schedules.
Site Selection and Feasibility Assessment
Oxygen Clinical Research evaluates research centre’s based on infrastructure, patient access, and readiness before involving sponsors or CROs. This pre-evaluation ensures smoother execution and higher success rates.
Task-Oriented Approach
Our task-oriented approach increases efficiency and consistency. Each team member is responsible for a specific role—for example, data entry staff focus solely on entering data without handling other tasks.
Timeline Tracking
We assist clients in setting, monitoring, and maintaining key study milestones and deadlines throughout the clinical trial process


